Powerful SaaS platform for the research industry.
Fast-track your decentralised clinical trials or observational studies.
Combining ResearchAppTM , our patient-friendly app and Healthbit IQTM, our study management and intelligence dashboard.
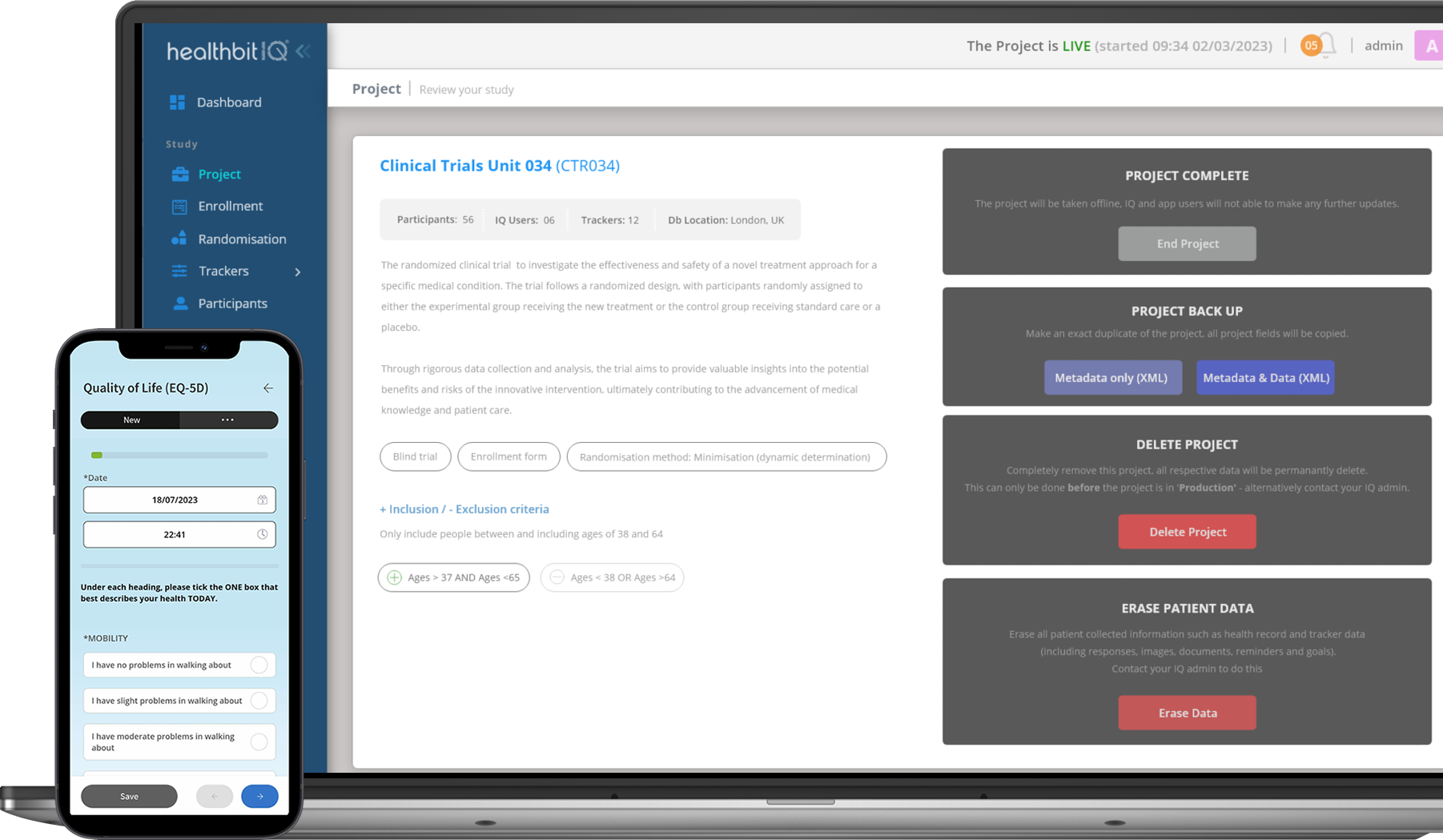
The Healthbit IQ Clinical Trials Platform
Our powerful technology empowers researchers, enhances participant engagement, and accelerates medical research. With the Healthbit platform, we’ve done the hard work to bring you easy-to-use tools that integrate seamlessly together and allow you to setup, operate and monitor your study.
Whether you’re conducting decentralized, hybrid trials or observational studies; seeking to improve participant engagement in studies or seeking compliant data capture capabilities; we have you covered.
We’re making research smarter, faster, and more inclusive.
Reduce Trial Complexities & Timelines
Our extensive SaaS platform facilitates the rapid customisation and deployment of the patient-friendly ResearchAppTM for decentralised trials and studies.
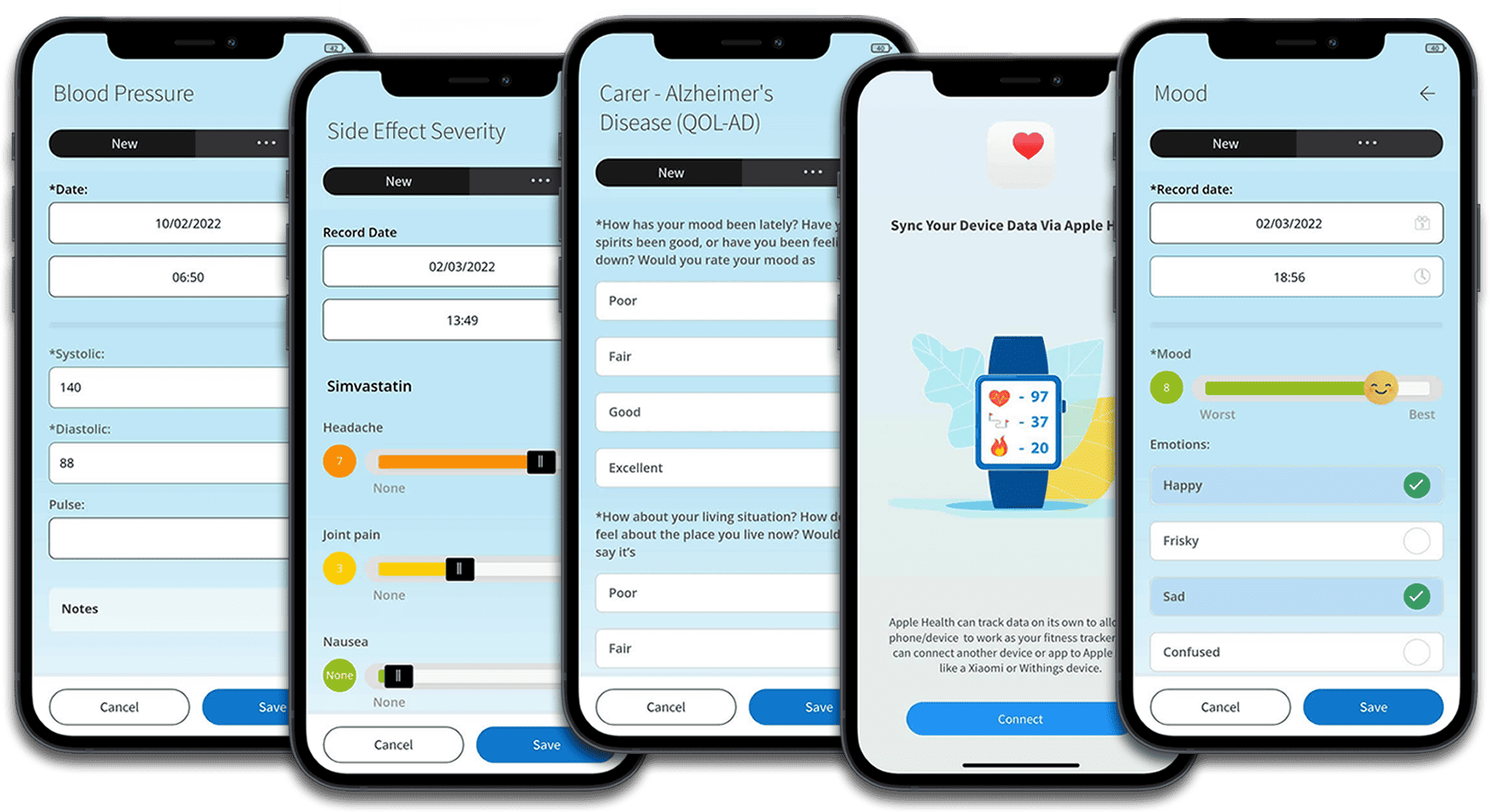
Capture everything: eCOA, ePROs, connected device data, symptoms, adverse events, health and lifestyle indicators, resource utilisation and more. Data is seamlessly captured, structured and made available in reporting dashboards. View insights, download data or integrate it via the API.
Accelerate study design with our extensive library of clinically validated ePRO/eCOA surveys, assessments and health trackers, or simply design your own.
Use the ‘drag and drop’ features to build and test custom surveys with advanced features, including:
- Validation & Branch Logic
- In-line calculations and scoring
- Image and video capture for AI driven data processing.
Healthbit IQ Platform Features
User Friendly Design & Functionality
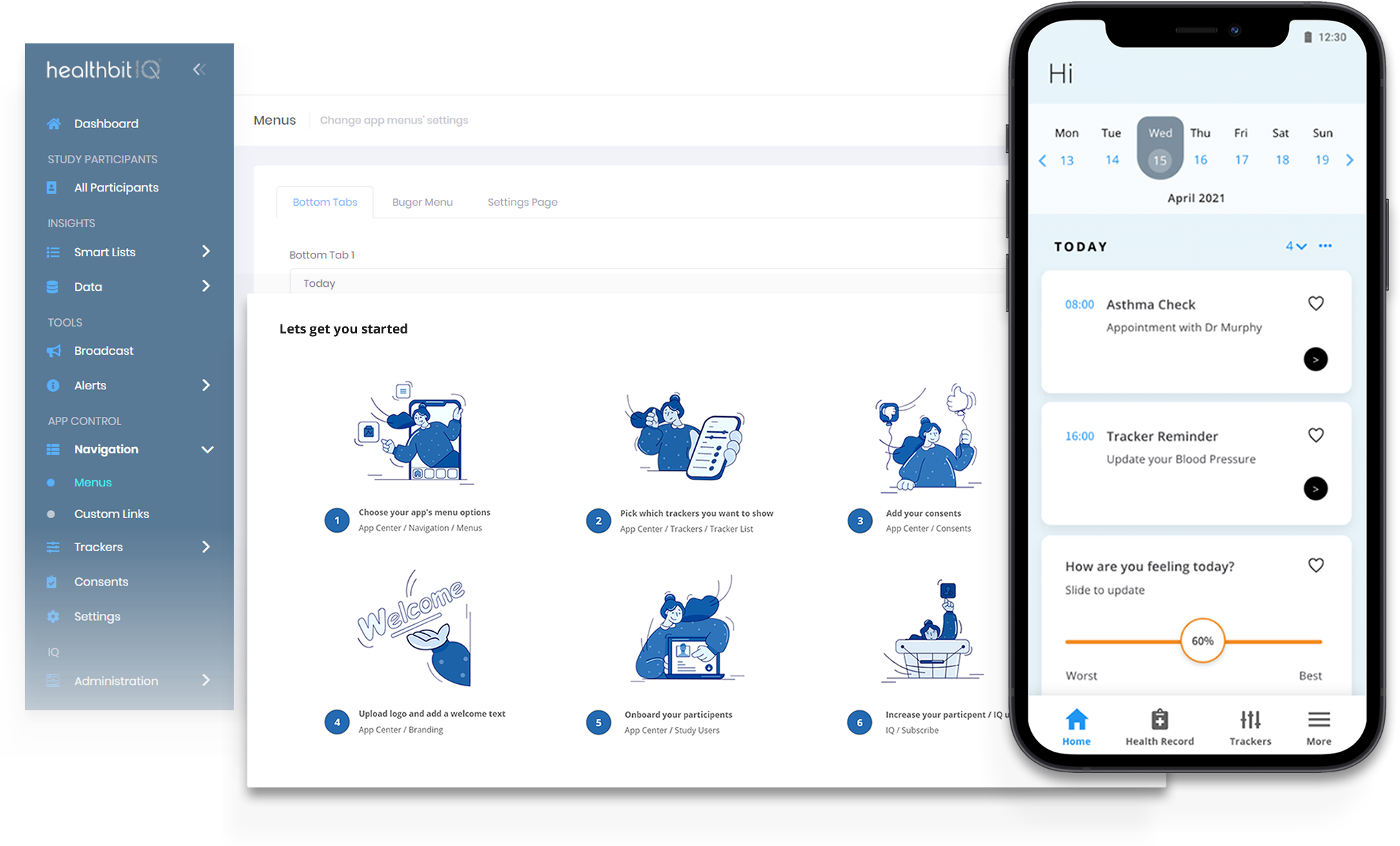
Intuitive workflow
Efficiently configure your trials with our user-friendly project setup feature. Define study parameters, manage workflow seamlessly, and set the stage for success.
From creating study protocols to designing data collection forms, our platform simplifies the setup process, ensuring trials start off on the right foot.
Data Collection and Compliance Made Easy
Put participants at the centre of the consent process and ensure study protocol compliance.
By utilizing the e-consents feature, researchers can streamline the consent process, reduce administrative burden, enhance participant understanding, and maintain compliance with regulatory standards. It promotes a participant-centric approach, improves data management, and ensures the ethical conduct of clinical trials.
Our e-consents feature adheres to regulatory standards and guidelines for electronic consent processes. The platform incorporates security measures to ensure data privacy, authentication of participants, and compliance with applicable data protection regulations.
Participants can access and review the consent forms through the Research App. This promotes transparency, participant engagement, and informed decision-making.
Built-In Randomisation

The built-in randomization module streamlines the randomization process. Researchers can easily configure randomization parameters within Healthbit IQ, allowing for efficient and automated allocation of participants to treatment groups. This helps maintain the scientific rigor of the study and enhances the validity of the research outcomes.
By enabling the randomization module researchers can eliminate the need for external statisticians solely for the purpose of randomization. This results in cost savings for research teams, allowing resources to be allocated to other important areas of the study.
Have your own data statistician?
No problem import randomisation assignments from popular statistical software packages such as Stata.
Randomisation methods include Simple Randomization, Stratified Randomization, Block Randomization, Cluster Randomization, Minimization and Stratified Blocked Randomization.
The choice of method depends on the study design, research question, and specific requirements of the study protocol.
‘Promoting Diversity in Clinical Trials’
Decentralizeed trials offer rapid patient data collection reducing time and cost and accessible to a more diverse participant base.
The platform has a versatile participant enrollment form that can be made publicly available (or password protected) so potential candidates can be screened for inclusion.
Partnering with patient support groups
Medical Research Charities and patient groups are often the source of unparalleled knowledge and information on specific conditions, and in many instances act as an invaluable and trusted intermediary between patients and the healthcare system.
We work with Patient Support Groups to grow their patient base and quickly build an organised patient registry. Data collected can contribute to a better understanding of the disease and help drive recruitment for clinical trials.
Rarer conditions affecting fewer people worldwide or localised conditions in certain geographical areas (which are often overlooked) – will benefit immensely from having a greater pool of participants to analyse and gain insights from.
Surveys & Validated Instruments
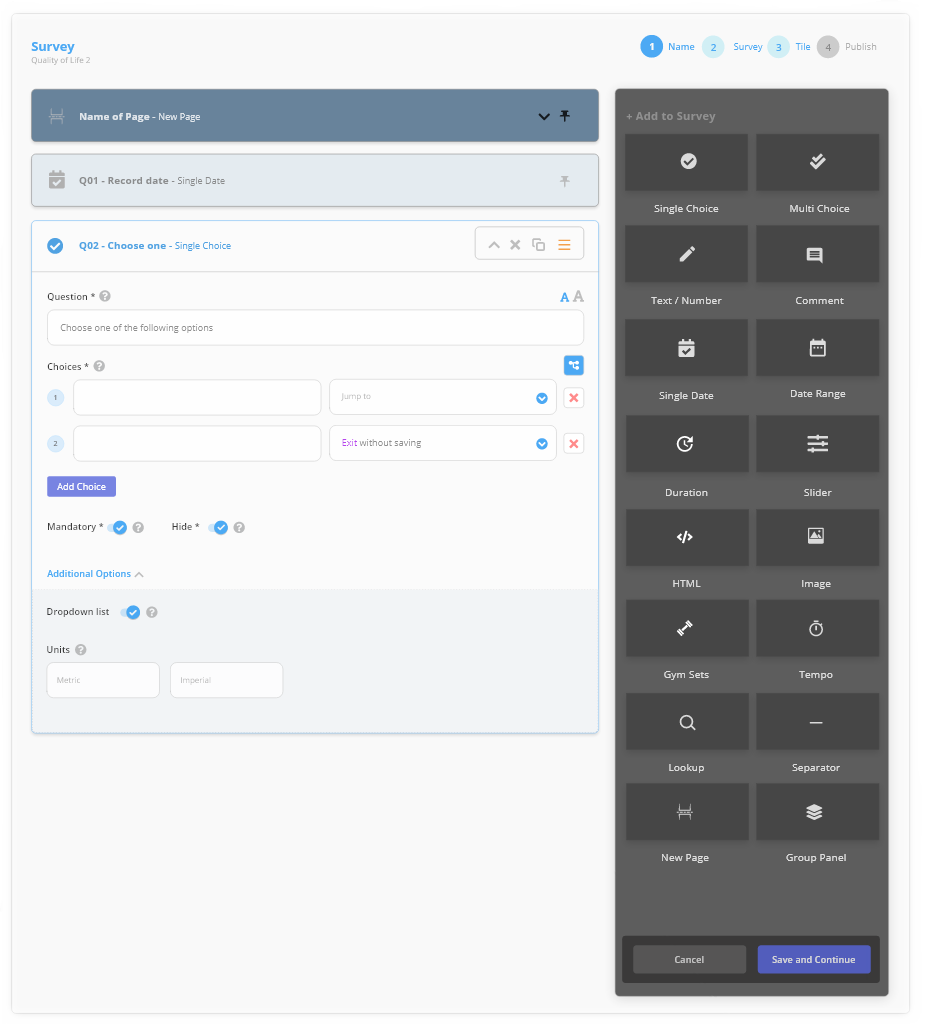
Clinically valid ePRO/eCOA surveys
Capture scientifically sound data via approved research instruments.
Extensive library of surveys and health & lifestyle trackers.
Connectivity with popular medical device and health apps (BYOD).
Or even build your own using customised surveys with advanced features, including calculations and update frequency.
Easley Create a Perfect App for Your Study
Research App is a powerful feature of our platform that empowers researchers to create a tailored and engaging mobile experience for trial participants.
You’re in control of everything from branding, consent screens, layouts and surveys.
Design custom surveys with our ‘drag & drop’ tools or select surveys to use from our extensive library; diaries, ePROs/eCOA, QoL, symptoms, health indicators, lifestyle, exercise, and more.
Real-Time data collection and monitoring
Integrating medical devices enables seamless data collection in real-time. By connecting with wearable devices, sensors, and other medical devices, our platform can capture objective measurements such as heart rate, blood pressure, glucose levels, activity levels, and more. This real-time data provides researchers with up-to-date and accurate insights into participants’ health status, improving the overall quality and timeliness of data collection.
This reduces the reliance on self-reported data, which can be subject to recall bias and variability. This enhances the integrity and validity of the trial data, leading to more robust and trustworthy research outcomes.
Simple Onboarding Of Users
- Generate secure login tokens automatically for participants.
- Participant identifiers are not required to keep data anonymized.
- Keep your data compliant by storing the data in either the UK,US or EU.
Boost Participant Engagement
- Our suite of tools are designed to keep participants actively involved in the research process.
- Set up automatic reminders to activate content or indicate when surveys require updating.
- We empower participants to stay informed, motivated, and committed to their role in the trial.
- Curated and scheduled content educates, informs and delights.
- Real- time engagement metrics, data visualisation and communication tools help you monitor your study and mitigate drop-out.
‘Self-Serve, Participant Monitoring & Alert System’
Providing study teams with a cost-effective option for monitoring participants in real-time to improve health outcomes and mitigate risk.
Surveys and health trackers can be assigned notification, by setting thresholds and alert escalation.
Complex dependencies can be used to trigger actionable alerts to reduce false alerts.
Examples:
- Real time tracking and alerting of adverse effects
- Identify patients at risk through smart alerts
Streamline Communications & Data Collection

Investigators can utilise the Televisits Engine to communicate with participants while collecting eCOA endpoint data.
Facilitate telemedicine visits and electronic Clinical Outcome Assessments (eCOA) through our integrated platform. Conduct virtual visits, capture patient-reported outcomes, and enhance the flexibility and convenience of participant engagement. Save time, reduce costs, and improve overall participant experience with seamless televisit and eCOA capabilities.
Personalized and adaptive interventions
Real-time data enables personalized and adaptive interventions based on individual participant needs. Researchers can monitor participants’ health trends and remotely provide tailored recommendations, interventions, or adjustments to treatment plans. This personalized approach improves participant outcomes and allows for more responsive and individualized care.
Real-Time Data Visualisation
Unlock the power of your trial data with our advanced analytics and visualization capabilities. Gain actionable insights, identify trends, and visualize data patterns through interactive dashboards and reports. Our data analytics tools help researchers make informed decisions, drive evidence-based conclusions, and accelerate the translation of research into clinical practice.
Customisable dashboards with rich interactive reports and structured encoded data (SNOMED CT\MedDRA) give you increased efficiencies.
Data can be exported or accessed via the API in real-time.
API built for developers by developers.
Easily Customise App Features, Branding & Menus
Empowering researchers to create a tailored and engaging mobile experience for trial participants:
Trackers
Connected Devices
Tracker Update Reminders
Patient Health Record
Medication Adherance Engine
Branding
Menus
Custom Links
Manage eConsents
Profile Questions
Forums \ Support Tools
Automatic Study Invites
Role-Based Access
Data Location for Cloud Storage (UK, US, EU)
Customisable Research App
You’re in control of everything from consent screens, layouts and surveys.
Maintaining Compatibility with Global Standards
We support a number of global dictionaries and standards to optimise data interpretation, compatibility and interoperability, such as SNOMED-CT, OMOP, CDISC, FHIR and others.





